Validation of dried blood spot biomarkers for identification of depressed patients with bipolar disorder misdiagnosed as having major depressive disorder
Conducted by the research team of Professor Sabine Bahn at the Cambridge Centre for Neuropsychiatric Research (CCNR), University of Cambridge (ccnr@ceb.cam.ac.uk).
Participant Information Sheet
Dear Participant,
We would like to invite you to take part in this research study. The purpose of this study is to test how well certain blood biomarkers can help us distinguish bipolar disorder from major depressive disorder. Biomarkers are biological indicators in our bodies that doctors can use to check our health or diagnose diseases. This study focuses specifically on people who have been recently (in the last 5 years) diagnosed with major depressive disorder by a medical professional and are currently showing signs of depression. These may include feeling depressed or low, getting less pleasure out of or being less interested in things you usually enjoy, or feeling tired more easily. We will ask you to complete an online questionnaire to collect information about you and your symptoms, and may request you to provide a fingerprick blood sample by post. Our aim is to create a blood test that can accurately detect bipolar disorder early, so that patients can receive the right treatment sooner.
Before you decide to take part, it is important that you understand what it will involve. Please take your time to read the following information carefully. You can contact a member of the team at ccnr@ceb.cam.ac.uk if you have any questions.
If you would like to download a copy of this Participant Information Sheet for your future reference, click here.
What is the aim of the study?
Our study aims to validate a panel of blood biomarkers for better diagnosing bipolar disorder, a condition often misdiagnosed as major depressive disorder, especially during episodes of low mood. Bipolar disorder affects about 1.3 million people in the UK, yet approximately 40% initially receive an incorrect diagnosis. Such misdiagnoses can lead to inappropriate treatments and an average delay of 5 to 7 years in receiving the correct diagnosis. Our focus is on a panel of 17 specific biomarkers identified in our previous research involving 241 patients initially diagnosed with major depressive disorder, 67 of whom were later found to have bipolar disorder. This biomarker panel aims to provide a more objective approach to diagnosing bipolar disorder, particularly in primary healthcare settings where the majority of misdiagnoses occur. Through this study, we hope to make a significant step towards reducing the high rates of misdiagnosis of bipolar disorder, leading to quicker treatment and better outcomes for patients.
Can I take part?
To be eligible for this study, you must be:
- Between 18 and 45 years of age
- Living in the UK
- Recently diagnosed with major depressive disorder (within the last 5 years) by a healthcare professional, such as a General Practitioner (GP) or a psychiatrist
- Currently experiencing at least mild depressive symptoms (this will be assessed when signing up for the study)
Who cannot take part?
- People experiencing suicidal thoughts will not be eligible to participate due to safety reasons. We would instead advise you to contact your GP or the Samaritans (http://www.samaritans.org/how-we-can-help-you/contact-us) by dialling 116 123. If you feel you are unable to keep yourself safe, call the NHS mental health line by dialling 111. If you urgently need help or support, call 999 or go to A&E. If you are unable to call or unable to get there by yourself, please ask someone to call on your behalf or take you
- People with a previous diagnosis of bipolar disorder
- People previously diagnosed with schizophrenia
- Anyone with a blood-borne illness (e.g. HIV infection or hepatitis)
- If you are currently pregnant or breastfeeding
Do I have to take part?
No, you don’t have to take part. The information presented here is designed to help you decide if you would like to take part in the study. If you decide to take part, you will be given a random ID code, which anonymises your responses in the online questionnaire and any blood samples you may provide in the future. You will also be free to withdraw from the study at any point by closing the browser, but this will not automatically remove any information you have already given from the database. If you wish for your responses and/or samples to be removed, you must email us at ccnr@ceb.cam.ac.uk and provide us with your ID code.
What is involved?
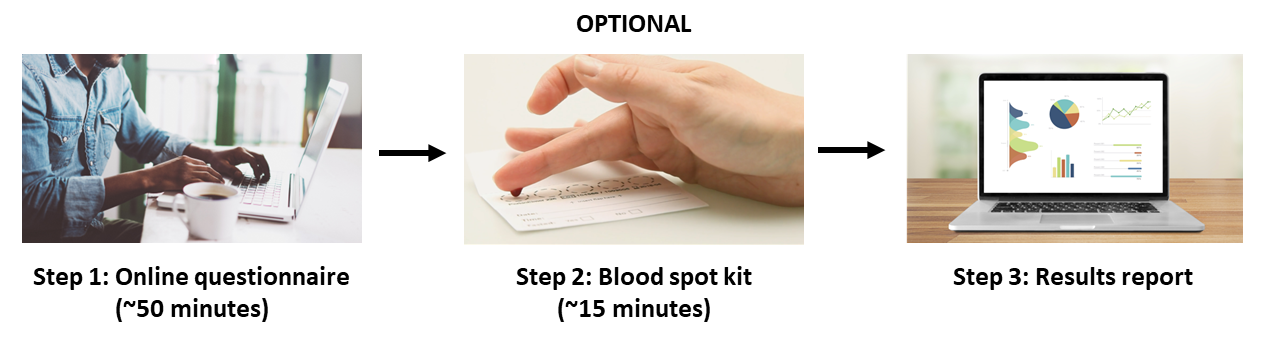
The steps involved in our study are summarised in the flowchart below. You will be asked to provide information about yourself and your mental health using an online questionnaire. Some participants will also receive a dried blood spot collection kit by post to provide a blood sample. At the end, all participants will receive a personalised results report based on their answers from the online questionnaire.
- What will the online questionnaire ask me about? The online questionnaire consists of 6 question sessions. These question sessions will ask you about your past and present mood, demographic details, lifestyle, personality, psychiatric history, general medical conditions, and other factors. The questionnaire assesses for symptoms of major depression, bipolar disorder, and other psychiatric conditions.
- What is the dried blood spot collection kit? The kit will provide you with all the materials you need to collect a dried blood spot sample at home. If you are selected to donate a blood spot sample, the kit will be mailed to your address. The kit takes approximately 15 minutes in the morning to complete. You will also be sent a prepaid envelope to return the kit to us for analysis once you have completed it. Please note that not all participants will receive a blood collection kit. After processing your answers to the online questionnaire, the study team will confirm by email if you are selected to receive a kit.
- How will I know if I have been selected to receive a blood spot collection kit? If you have been selected to donate a blood sample, we will get in touch with you by email so that you can tell us where to send your blood spot collection kit. In that case, it may take anywhere from several days to several weeks after completing the questionnaire before you hear from us.
How much time will this take?
Most people complete the questionnaire within an hour. After starting, you will have two weeks to complete it. We strongly recommend answering all questions in one go, but you can pause and resume where you left off, provided you reopen the questionnaire in the same browser and with the same cookies enabled. Please note that, unfortunately, we will be unable to retrieve any answers that have been lost before the questionnaire is completed.
If selected, you will also need another 15 minutes to provide a dried blood spot sample.
What if I no longer wish to participate?
Your participation is completely voluntary, and you can stop your participation at any time. You do not need to provide a reason, although any feedback on your experiences with the study would be greatly appreciated. If you wish to stop your participation, email us at ccnr@ceb.cam.ac.uk with your ID code so we can remove your data and any samples you may have provided. We will also stop any further communication with you about the study. However, please note that it will not be possible to retract your data from materials which have already been published by the time you withdraw your consent.
What are the possible benefits of taking part?
Once the study recruitment phase has been closed, you will receive a personalised report to your email indicating whether you are likely to have bipolar disorder and providing further relevant information. Please note, this report is only an indication and does not provide a diagnosis. We encourage participants to discuss their report with a healthcare professional if they wish to do so.
We hope that by answering the online questionnaire and reading your report, your participation may help you develop a deeper understanding of your mood-related experiences. Please note that your participation in the study will not affect the medical care you receive.
Your participation will also benefit academic research into mental health and may help in the development of a blood test for aiding the diagnosis of bipolar disorder.
- What will my report be based on? The report will be based on your answers from the online questionnaire and our machine learning algorithm that can distinguish bipolar disorder from major depressive disorder in people with current depressive symptoms. The algorithm was developed in our previous study, and was 84% accurate in detecting bipolar disorder when compared to a diagnostic interview developed by the World Health Organisation, called the Composite International Diagnostic Interview. You can find more details about the algorithm here.
- Will the blood marker results be included in the report? No, blood biomarker results will not be considered in the report, even if you have donated a blood sample. This is because the blood test is still under development.
- Can your algorithm distinguish subtypes of bipolar disorder, such as bipolar I or II? No, the algorithm will only indicate how likely you are to have bipolar disorder on a scale from 0 to 100%, without specifying its subtypes. It will also state which of your answers contributed most to this prediction. It is important to understand that the result from the algorithm is not a diagnosis. An accurate diagnosis can only be made by a medical professional in-person.
- How long will it take to receive my results report? All study participants will receive their results reports at the same time, once the study recruitment phase has completed. We estimate that the recruitment phase will take about 3 months to complete.
What are the possible disadvantages and risks of taking part?
We have tried our best to minimise any disadvantages or risks of taking part. However, you should be aware that your participation requires the following:
- Time commitment: The study requires about an hour of your time commitment to complete the online questionnaire. Additionally, if you are selected to donate a blood sample, it will take you an extra 15 minutes or so to collect it.
- Personal questions: There is a chance that some of the questions in the online questionnaire will make you feel uncomfortable. We need all the answers to the online questionnaire for the algorithm to generate the personalised report, and so you need to make sure you are happy to answer questions about your mental and physical health before starting as you will not be able to skip any questions.
- A small pinprick: If you receive a blood collection kit, you will need to make a small pinprick to your finger. This will involve minimal pain or discomfort.
- Personalised feedback: You will receive personalised feedback after the study. Your final report will tell you whether your experiences may indicate you have bipolar disorder. You do not need to read your final report if you do not wish to receive this feedback. Bear in mind that this outcome is not a diagnosis and for a full assessment of your mental health you will need to see a medical professional.
If completing any part of the study upsets you in any way, we recommend that you stop and contact a healthcare professional such as your GP, or seek support via the following links:
- The NHS recommended list of mental health helplines: https://www.nhs.uk/nhs-services/mental-health-services/
- The Samaritans: https://www.samaritans.org/ (or call 116 123 for a free 24-hour helpline)
If you feel you are unable to keep yourself safe, call the NHS mental health line by dialling 111. If you urgently need help or support, call 999 or go to A&E. If you are unable to call or unable to get there by yourself, please ask someone to call on your behalf or take you.
Will I be compensated for my participation?
Unfortunately, we are unable to provide any financial compensation for your participation in the study.
In addition, we’d like you to note that the research we conduct with your dried blood spot (if you provide one) and with the data arising from your participation in the study may result in inventions or discoveries. These could become the basis for new products such as diagnostic or therapeutic tools. These inventions and discoveries may be of potential commercial value and may be patented and licensed. You will not receive any money or other benefits derived from any commercial or other products that may be developed from the use of your dried blood spot and/or study data.
Will my data be kept confidential?
Your personal data (name, email address, and other contact information) will be stored securely and will be accessible only to the researchers working on the study. Your research data (i.e. data from your questionnaire and potentially the blood sample) will be anonymous. This means it will be marked only with a code and will not be linked to any identifying information. Please note that your data may also be used to support other research in the future, and may be shared anonymously with other researchers.
Only in exceptional circumstances, in which we believe you are at risk of harming yourself or others, we may be required by law to breach this confidentiality by contacting your GP or emergency services.
Additionally, the survey platform we use, Qualtrics, will collect your IP address to automatically save your responses, allow you to return to the survey later, and prevent multiple submissions and submissions from outside the UK. This data will be stored securely in a password-protected file and will not be used for any other purpose. We will delete the IP address data once study recruitment is completed.
If you would like to know more about the use of personal data, please refer to the University of Cambridge's guidelines (https://www.information-compliance.admin.cam.ac.uk/data-protection/research-participant-data).
What will happen to the results of the research project?
Study results may be presented at scientific conferences and may be published in peer-reviewed scientific journals. Any presented or published data will be completely anonymous. You can follow updates by checking our website (http://ccnr.ceb.cam.ac.uk/), and Twitter (https://twitter.com/Bahn_lab) and Facebook (https://www.facebook.com/Cambridge-Centre-for-Neuropsychiatric-Research-741364285972168/) pages.
With this study, we also hope to take a big step towards developing a diagnostic tool that can be used by patients.
Who is conducting this research?
The study is carried out by the Cambridge Centre for Neuropsychiatric Research at the Department of Chemical Engineering and Biotechnology, University of Cambridge (http://ccnr.ceb.cam.ac.uk/).
Who is funding this research?
The study is funded by the Stanley Medical Research Institute (Bethesda, USA), a non-profit organisation supporting research on the causes and treatments of schizophrenia and bipolar disorder (http://www.stanleyresearch.org/about/), and by the Medical Research Council (London, UK), a public organisation dedicated to improving human health through research (https://www.ukri.org/councils/mrc/).
Ethical review of the study
This study was reviewed by the University of Cambridge Human Biology Research Ethics Committee, reference number HBREC.2024.02.
Insurance
Adequate provision is made for insurance or indemnity to cover liabilities which may arise in relation to the design, management and conduct of the research project.
What should I do if I have more questions?
If you have any questions or need more details about this study, please visit the Frequently Asked Questions section on our website or contact us at ccnr@ceb.cam.ac.uk.
Thank you for considering participating in our research study.
Principal Investigator:
Professor Sabine Bahn, MD, PhD, MRCPsych, FRSB
Researchers:
Jakub Tomasik, PhD
Nayra Martin-Key, PhD
Erin Funnell, BSc
Jihan Zaki, MPhil